
දිනපතා සත්ය කරුණු දැන ගැනීමට අපගේ WhatsApp චැනලයටමෙතනින් එකතුවන්න.
There has been widespread international attention regarding concerns raised over several infant formula products manufactured by Nestlé. These concerns stem from reports suggesting that certain batches of infant formula could potentially contain a harmful substance capable of causing food poisoning. Fact Crescendo conducted an examination of the accuracy of these claims, and the findings are outlined below.
Social Media Posts :
A Facebook post currently circulating on social media claims that Nestlé NAN milk powder is being recalled globally after laboratory tests allegedly detected the presence of a toxic substance known as cereulide. The post further implies that the consumption of the product poses a serious health risk to infants.

Another social media post published on the same subject was later updated, further drawing public attention to the issue.

In response to numerous requests received from social media users worldwide, particularly from Sri Lanka, regarding the alleged risks associated with Nestlé infant formula products, Fact Crescendo initiated a detailed fact-checking process to verify the accuracy of these claims. The steps taken in this investigation are explained below.
Explainer :
International media reports
Reports that Nestlé had recalled several infant formula products distributed globally under different brand names became a matter of international concern following coverage by multiple foreign media outlets.
According to BBC News, Nestlé initiated a global recall of several infant formula products as a precautionary measure amid concerns that certain batches could contain a toxic substance capable of causing food poisoning. The report, citing Nestlé, states that some batches of infant formula sold worldwide may not be safe for infant consumption.
The company further noted that although the affected milk powder, which may contain cereulide, had been distributed globally, there have been no confirmed reports of infants falling ill after consuming the product. Nestlé stated that the recall was initiated voluntarily as a precautionary step.
Another international media report states that the recall followed an internal investigation conducted at Nestlé’s production facility in Nunspeet, the Netherlands, which prompted the company to take preventive action.
Foreign media also reported that several Middle Eastern countries, including the United Arab Emirates, have recalled certain stocks of Nestlé infant formula as a precautionary measure. According to these reports, recalls have taken place in the United Arab Emirates, Bahrain, Egypt, Iran, Kuwait, Oman, Qatar and Saudi Arabia.
Citing the UAE Pharmaceuticals Agency, the reports state that the recall applies to the following product categories: NAN Comfort 1, NAN Optipro 1, NAN Supreme Pro 1, 2 and 3, S-26 Ultima 1, 2 and 3, and Alfamino.
Meanwhile, Reuters, quoting the UAE Pharmaceuticals Agency, reports that there have been no confirmed cases of children becoming ill after consuming the affected product groups. Several other international media outlets have also published reports on the matter, which can be found here, here and here.
Nestlé’s clarification
Nestlé has issued a series of clarifications regarding the situation involving its infant formula products. The company stated that all infant formula products were tested after an issue was identified with an ingredient supplied by a third-party supplier.
Nestlé further explained that all arachidonic acid (ARA) oil and related oil blends used in potentially affected infant formula products had undergone testing. According to the company, no illnesses among infants have been reported to date in connection with the products.The company advised that parents and caregivers should consult a pediatrician if there are any concerns regarding a child’s health. Additionally, Nestlé instructed that the affected products should be discontinued as a precautionary measure, even in the absence of symptoms.
Which countries and brands are affected by the recall?
According to Nestlé, the infant formula recalls currently apply to specific products distributed in several countries. The list of affected countries and product brands is outlined below.
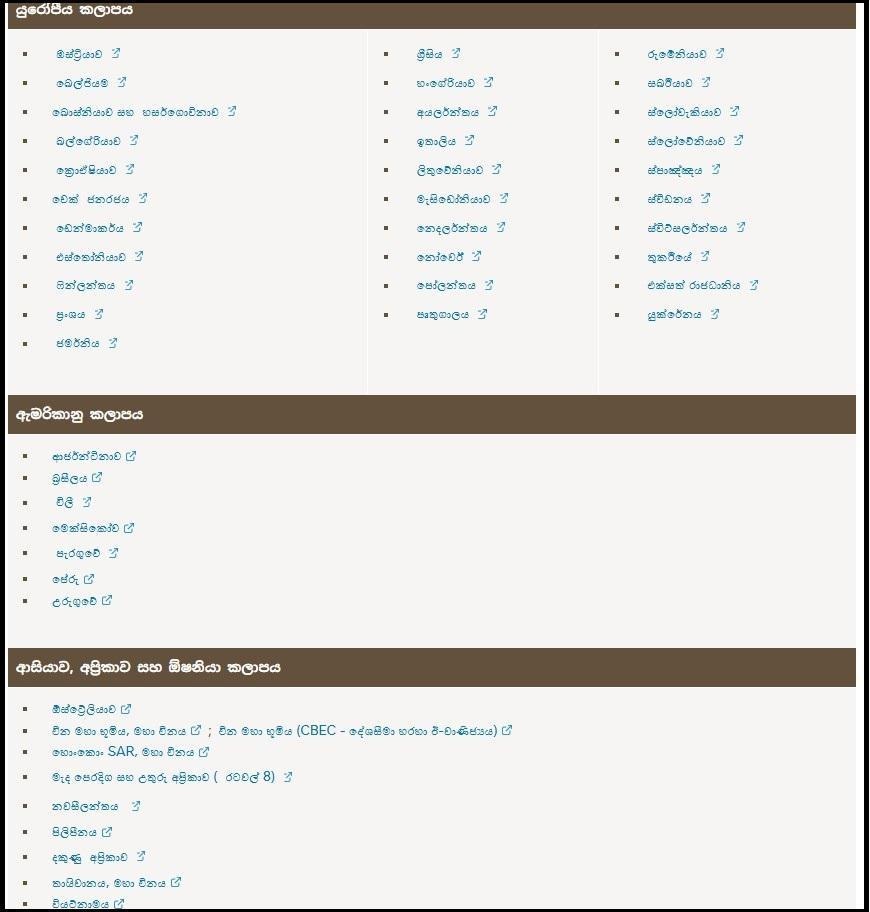
Another international media report also addressed the matter in this way. However, as regional countries such as Sri Lanka and India were not listed among the affected markets, Fact Crescendo contacted Nestlé Lanka to ascertain whether local consumers are impacted by the situation.
Situation in Sri Lanka – Nestlé Sri Lanka’s response
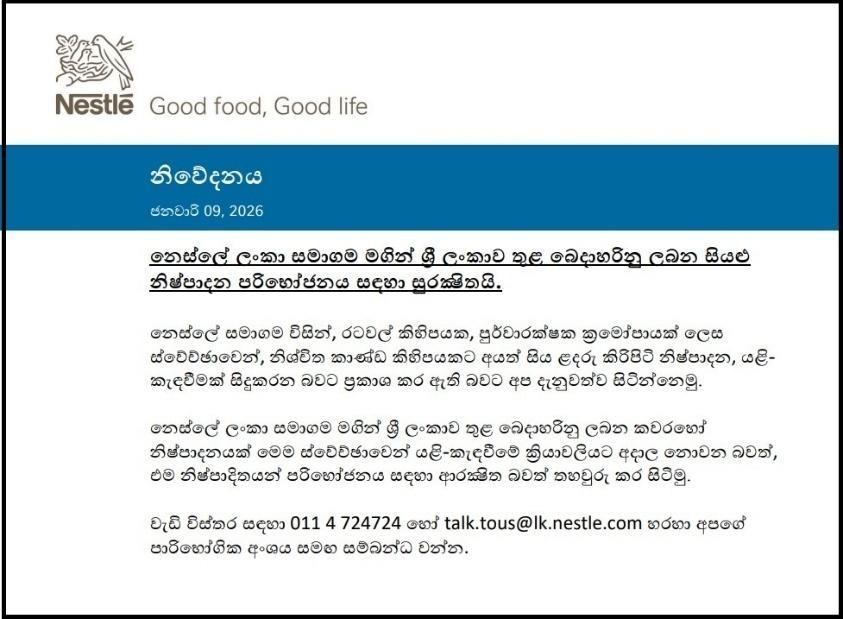
Nestlé Lanka confirmed that all infant formula products distributed by the company in Sri Lanka are safe for consumption. The company further stated that none of the products currently marketed by Nestlé Lanka in the country are subject to the voluntary recall process announced internationally.
Nestlé Lanka has also issued an official statement clarifying the situation. The relevant statement is presented below.
Can recalled products be found in stores selling imported milk powder?
While several of the infant formula products included in the recall are not officially distributed in Sri Lanka by Nestlé, some affected brands are available locally through parallel imports, foreign distributors and online sellers. As a result, these products are not included in the recall list issued by Nestlé Lanka.
However, certain recalled brands, such as SMA can be purchased in Sri Lanka through unofficial import channels. Therefore, parents and caregivers purchasing imported infant formula are advised to consult international recall notices relevant to the country of origin before use.
How can you check whether a product is affected?
In its global notification, Nestlé stated that arachidonic acid (ARA) oil is used in a range of infant nutrition products sold worldwide. As a result, the specific brands and product variants affected differ from country to country. Accordingly, the company has announced that details of affected products will be published on the official Nestlé brand websites in the respective countries.
Nestlé advises consumers to verify whether a product is affected by checking the batch number and related product information available on the relevant country-specific Nestlé website.
For instance, in the United Kingdom, details of recalled infant formula products and their batch numbers were published on Nestlé’s official UK website like this. Similarly, the Food Standards Australia New Zealand (FSANZ) has issued notifications identifying the affected product groups in Australia and New Zealand like this.
If a product is confirmed to be subject to a recall notice, consumers are advised to discontinue its use immediately and follow the instructions provided in the recall announcement. Nestlé has further stated that information regarding recalls, refunds and consumer guidance can be obtained through the relevant Nestlé brand websites.
What is cereulide?
Cereulide is a toxin produced by the bacterium Bacillus cereus and is known to cause symptoms of food poisoning, including vomiting and abdominal cramps. According to the Food Standards Agency (FSA), cereulide is heat-stable and difficult to destroy through cooking, boiling or during the manufacturing process of infant formula.
Nestlé’s full statement regarding the affected infant formula products is available through its official communication channels.
Join us to learn about our fact checking investigations.
Facebook | Twitter | Instagram | Google News | TikTok | YouTube

Title:Is Sri Lanka among the countries recalling the questionable Nestle infant formula?
Fact Check By: Sagarika ChathumaliResult: Insight






