Subscribe to our WhatsApp Channel
About two years ago, the import of substandard drugs into the country through the National Medicines Regulatory Authority of Sri Lanka, which were unsuitable for use, and the allergies and complications that patients experienced as a result of administering those substandard drugs, became a topic of great controversy in society. Among these drugs, great attention was paid to the immunoglobulin drug, and in light of the problematic situation that arose, we took steps to investigate the published social media posts indicating that this drug, which had been withdrawn from use, was being reused.
Social Media Posts :
Social media posts were circulated stating that permission had been granted to re-import a substandard medicine brought by former Minister Keheliya Rambukwella.
Here’s how it was widely shared among social media platforms.
We took steps to check the accuracy of these shared posts.
Fact Check :
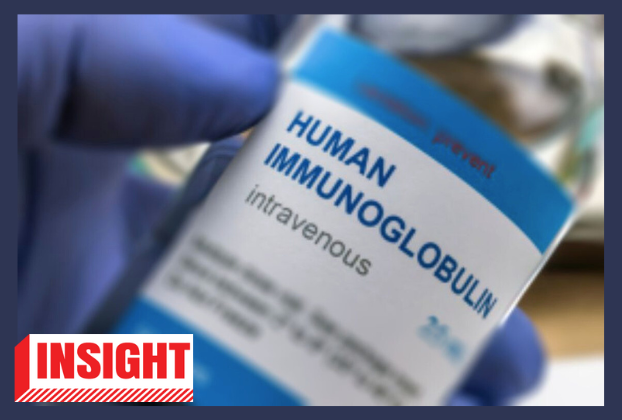
Human immunoglobulin
Intravenous immunoglobulin (IVIG) is an immune-boosting treatment given to people with neurological disorders and those born with a weak immune system. Since it is made from donated human plasma, it is manufactured by high-quality pharmaceutical companies under strict standards, as there is a risk of transmitting infections if not properly tested and purified. More information regarding it here, here, and here.
A misleading statement from Hiru News
On May 31st, a news report on Hiru Media news stated that, Dr. Chamal Sanjeewa, Chairman of the Doctor’s Trade Union Organization for Medical and Civil Rights, alleges that the Medical Supply Unit has informed hospitals to reuse some of the substandard immunoglobulin drugs that been withdrawn from use. It further stated that the doctor has filed a complaint to the Commission to Investigate Allegations of Bribery or Corruption regarding this.
The media outlet states that Mr. Keheliya Rambukwella has also been summoned to court for importing the immunoglobulin drug mentioned here, into the country. You can watch that video here.
However, Dr. Chamal Sanjeewa, mentioned in this news, stated to the media, that after the use of the immunoglobulin drugs imported during the time of the questionable Keheliya Rambulwella was removed, the same medicine, imported from another company was used for the second time. It was also removed from use because it was found that the drug containes various bacteria, toxins, glass beads, and various other componenets. But he mentioned that the Medical Supply Unit informed hospitals to use the same medicine again. You can refer that to here.
However, since the Hiru News media network reported that the immunoglobulin medicine imported by former Minister Keheliya Rambukwella had been imported again, comments were exchanged on social media, which has now become questionable, stating that the same medicine was imported by the current government.
“It is not Human immunoglobulin imported by Rambukwella”- Health Minister Nalinda Jayatissa
Minister of Health Nalinda Jayatissa pointed out at a media briefing held at the Government Information Department on June 5th that, as the Immunoglobulin drug is an important drug given to patients, it was approved through a cabinet paper and was imported from a reputable company in India by a reputable supplier in the country, not the same drug that was imported by Keheliya Rambukwella. He further states that as several complaints were received, the National Medicines Authority conducted an investigation into it, and the remaining stock was temporarily held. Based on the reports received from the National Medicines Regulatory Authority Laboratory in Sri Lanka as well as from Indian laboratories, a group of local medical specialists recommended that this drug be used again. However, the Minister pointed out that this is by no means the immunoglobulin medicine associated with Keheliya Rambukwella.
Immunoglobulin medicine related to Keheliya Rambukwella
The information revealed by the media in this regard in 2023 stated that, among the medicine imported to the country using the strategic method of Certificate of Waiver of Registration (WOR), which could bypass the lengthy procurement procedures, in the face of the economic crisis, the drug called Intravenous Immunoglobulin (IVIG) was included.
Reports of adverse reactions to the drug Intravenous Immunoglobulin (IVIG) distributed to government hospitals were reported from the Matale District Hospital and the Colombo National Hospital and two patients at Matale DGH have developed anaphylaxis (a life-threatening allergic reaction) after receiving this IVIG injection. Many serious allegations have been revealed regarding the way this drug was imported into the country and distributed. Jamuni Kamantha Thushara, Chairman of the Citizens’ Power Against Bribery, Corruption and Waste, had complained that this medicine had been imported using forged documents prepared with fake official seals and called for an investigation into the matter. Later, this complaint was also filed by medical associations, and the Ministry of Health has also made the same allegation and requested an investigation.
Accordingly, the manufacturer is Livealth Bio Pharma Pvt Limited in Gujarat, India. The local representative is a company called Isolez Biotech Pharma AG.
Former Health Minister Keheliya Rambukwella has carried out an emergency procurement process. Police investigations have revealed that the stock of human immunoglobulin vaccines purchased from a local supplier through this process was manufactured in this country by the aforementioned supplier. The Criminal Investigation Department also revealed in court that this supplier had manufactured the drug in his own factory using fruit juice manufacturing machinery. It has also been revealed that there is no high-tech factory in the country to produce a vaccine like immunoglobulin.
Accusations have also been made that the defendants, including Keheliya Rambukwella, who have been charged in connection with this incident, have criminally misappropriated one billion rupees of government funds. You can view more information here and here. Archived Link| Archived Link
Currently importing Immunoglobulin drug
Doctor Ananda Wijewickrama
The Chairman of the National Medicines Regulatory Authority, Specialist Dr. Ananda Wijewickrama, had issued a statement regarding the news article published in the June 1st Mawbima newspaper, with the headline “Discontinued immunoglobulin drug have returned” (“භාවිතයෙන් ඉවත් කළ ඉමියුනොග්ලොබියුලින් කුප්පි යළිත් ඇවිත්”), and the social media post circulating linking to this news article with the caption, “The Ministry of Health informs to distribute the substandard drugs to patients” (විෂ එන්නත රෝගීන්ට බෙදන්න, යැයි සෞඛ්ය අමාත්යාංශය දැනුම් දෙයි) .
It states that after the incident involving Mr. Keheliya Rambukwella, a company called ICHOR Biologics (Pvt) Ltd imported a drug called Immunoglobin, manufactured in India, into the country, and that registration for it in Sri Lanka was granted in April 2023. Since research conducted in connection with a complaint received regarding this immunoglobulin drug, revealed a problematic situation regarding the condition of the drug, the Safety and Risk Assessment Subcommittee has advised the Medical Supplies Division to remove the drug from use. Later, further research into this matter has revealed that the medicine was of proper standards. This has also been confirmed by quality research conducted on the drug by the Central Drug Quality Control Agency of India. After considering those reports, the Safety and Risk Assessment Subcommittee, consisting of specialist doctors, decided that the drug could continue to be used. It further stated that the recommendation to reuse this drug was made after the specialist and physicians of the Safety and Risk Assessment Subcommittee, considered all the relevant factors.
To further confirm how this drug was approved for reuse, when the Medical Supplies Division was notified by the Safety and Risk Assessment Subcommittee to remove this drug from use, we contacted Dr. Ananda Wijewickrama through telephone.
He points out that since this drug is produced from plasma fluid, there is a high risk of allergic reactions, and although prompt actions were taken due to complaints received, research are being continued. He mentioned that the research were also done by the Indian Central Drug Quality Control Agency and it was revealed that the drug meets the proper standards.
However, he pointed out that among the complaints they recieved there was a suspicion that a patient died due to the drug, but the research still does not confirm that the death occured due to the drug.
Journalist Sajeewa Wijeweera
Journalist Sajeewa Wijeweera had reported on his website that the drug Immunoglobulin had been temporarily withdrawn from use by a circular issued by the Medical Supplies Division on 10-09-2024 due to various allergic reactions and complications in patients after its use in hospitals in Sri Lanka.
It has been decided that the drug contained bacterial toxins and visible contaminants, after samples of the drug from various hospitals were brought to the Medical Supplies Division and was tested by the Drug Risk Assessment Sub- Committee of the National Medicines Regulatory Authority in the National Drug Quality Laboratory. Accordingly, it has been determined to be unsuitable for use according to the criteria of the British Medicines Code.
The Medical Supplies Division has issued a circular dated 2024-10-23 stating that the drug should be permanently withdrawn from use. However, the stocks brought to each hospital have been safely stocked until the National Dangerous Drugs Control Board arrived and took them away after documentation.
After being notified to reuse the stock of medicines that had been removed from the ‘Swastha’ database of the Medical Supplies Division, they question how the Immunoglobulin drug was revoked when there are visible waste matters in the drug even after confirming that bacterial toxins were clearly identified in the Immunoglobulin drug imported from India. The web article posted by journalist Sajiwa regarding this is here. Archived Link
අපගේ කරුණු විමර්ෂණ පිළිබඳ තොරතුරු දැන ගැනීමට එක්වන්න
Facebook | Twitter | Instagram | Google News | TikTok

Title: An explanation regarding the controvery over the Immunoglobulin drug!
Fact Check By: Factcrescendo TeamResult: Insight