As new variants of COVID19 continue to challenge nations around the world, the vaccination drive against this deadly virus, which began around a year ago, continues to be a much-discussed topic as well. While WHO has approved a number of vaccines with two doses, with the evolving nature of the virus, some experts have highlighted the importance of having a third dose or booster dose of vaccination.
As some countries of the world, along with Sri Lanka, have started administering the booster dose, misinformation concerning the same has also started spreading. This is our investigation regarding such a misleading claim about the booster shot of Pfizer vaccine.
Social Media Posts
A post related to the booster dose of Pfizer vaccine started spreading via social media platforms with a link to a website. It stated that the Food and Drug Administration (FDA) of the USA has rejected the Covid19 booster shot for the age group of 16-65 years, over increased risk of heart inflammation, as seen below.
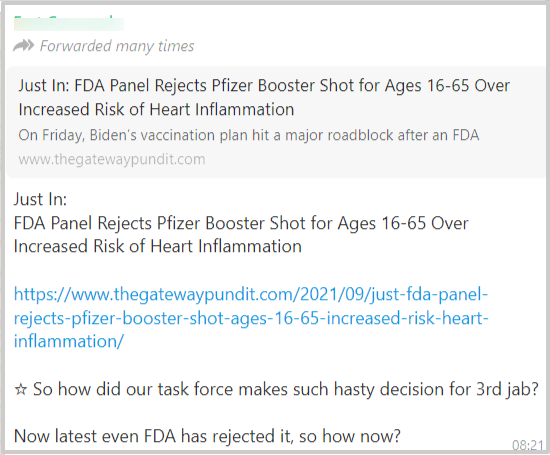
Due to the viral nature of this post, we decided to do a fact check on this.
Fact Check
First, we analyzed the link associated with the viral claim on social media, published by a website called thegatewaypundit.com
The article says that “FDA panel in September voted against the suggestion of giving booster dose to the population (16 to 65 age) who are in good health due to the insufficient data from incomplete clinical trials and the potential risk of heart inflammation, especially among young men” (The related screenshot is pasted below)

Global media reported on 18th September that while the FDA advisory panel had backed Pfizer booster shots for people 65 and older and for anyone with a high risk of severe COVID, the panel also recommended against approval of a booster shot for people 16 and older. NPR | NYT
However, in the statement dated 22nd September, FDA stated that reason behind approving booster vaccine to 65+ age group was taken based on available data and evidence that time and also mentioned that in addition even individuals who are at high risk of COVID19 in the age group of 16-64 and individuals who are at frequent exposure to COVID19 are also allowed to have a booster dose as seen below.

FDA`s related advisory panel was given the responsibility to decide whether the vaccine was needed or not needed to all above 16. However, the advisory committee decided that there was no sufficient evidence at that time to give vaccines to all individuals above 16. More details can be read from here.Archived.
However, after two months, on the 19th of November FDA gave permission to administer Pfizer and Moderna vaccines as a booster shot to everyone who are aged 18 or above in age. In this statement FDA did mention about the risks of heart inflammations, but they emphasized that benefits are overcoming the potential risks. (The related screenshot is seen below)

On November 19, American CDC (Centre for Disease Control and Prevention) also gave their permission to have a booster dose to people who are 18 years old or above as seen below.

Incidentally, Pfizer is the first covid19 vaccine, which received the approval from FDA in August 2021.
Also it is reported that “Heart inflammation is an exceedingly rare risk of both the Pfizer and Moderna vaccines, and it is more commonly seen in young men or boys and that it’s difficult for clinical trials to detect such a rare problem. And public health officials have repeatedly stressed that COVID-19 itself can cause heart inflammation at higher rates than the rare cases caused by the vaccine.”
However, there have been few reports of increase in cases of heart inflammation in Australia linked to the Pfizer vaccine and Taiwan suspending 2nd doses of Pfizer for teens over health risk.
The viral social media claims in Sri Lanka about the Pfizer vaccine and booster dose, is being shared at a time when the country has been administering the booster dose for frontline health workers from the beginning of November and for people above the age of 60 people from few days ago. Details about this can be seen here and here.Archived
To know more details about this issue, we contacted Professor Neelika Malavige Head – Department of Immunology and Molecular Medicine, University of Sri Jayewardenepura. She stated that the details mentioned in the viral social media post were not true and without any substantial test data. She further added that currently, both the Pfizer and Moderna vaccines are recommended by FDA as a booster dose US adults as seen here
We also contacted to the Professor Padma Gunarathna, president of Sri Lanka Medical Association (SLMA). She said that SLMA had requested from government to administer the booster dose for people above 60 years, frontline workers and immune suppressed personnel.
Here is a video where Dr. Kate O’Brien of WHO explains on the evidences so far about safety and effectiveness of COVID-19 booster shots. Director General of WHO, Dr.Tedros Adhanaom also recently stated that the booster dose of vaccine is not the need of the moment as poor countries of the world suffering without even first dose of vaccination and the world should focus on giving vaccination to poor countries rather than focusing on booster dose. The video can be watched from here.
Follow us and stay up to date with our latest fact checks.
Facebook | Twitter |Instagram | Google News
Conclusion:
According to our investigation, we found that FDA didn’t reject the Covid19 booster shot for age 16-65 over increased risk of heart inflammation in September. FDA didn’t’t approve the Pfizer booster dose back then as there were no sufficient evidence to administer booster dose to age group 16-65 back then. However, on the 19th of November, FDA as well as CDC, approved the booster dose of Pfizer and Moderna for all adults above 18 years. Incidentally, Pfizer was the first COVID19 vaccine to receive FDA approval.
There have been certain cases of heart inflammation related to Pfizer vaccine however this is an exceedingly rare risk and FDA also states in an official statement that while there are risks of heart inflammations, the benefits are overcoming the potential risks.

Title:Did FDA reject the Pfizer booster shot for ages 16-65 over increased risk of heart inflammation?
Fact Check By: Kalana KrishanthaResult: Misleading